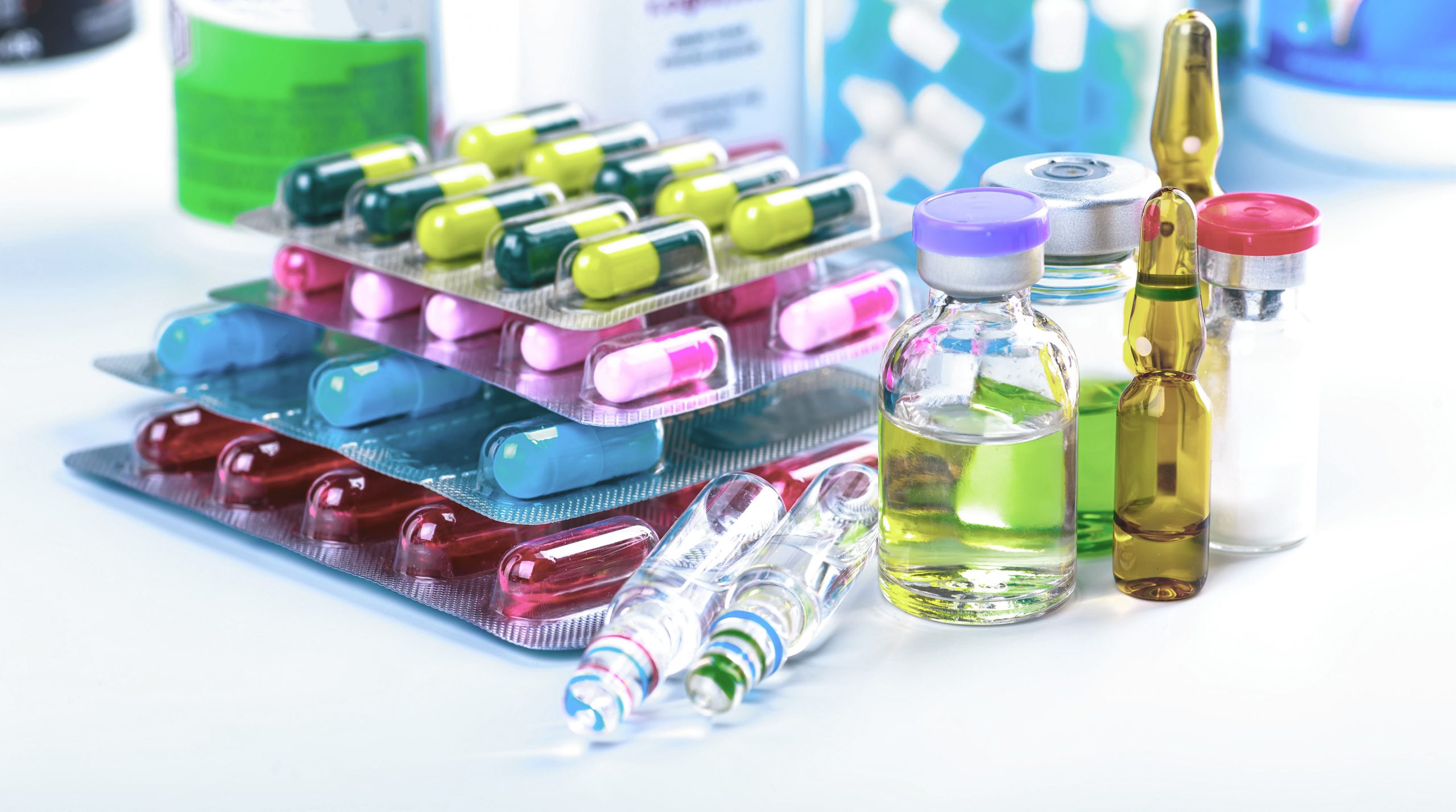
EPA Hazardous Pharmaceutical Waste Management Explained – Expert Solutions From Secure Waste
Managing hazardous pharmaceutical waste is a critical responsibility for healthcare organizations due to the environmental, regulatory, and safety risks associated with it.
The Environmental Protection Agency introduced a dedicated rule for hazardous pharmaceutical waste management to address gaps in existing hazardous waste regulations and provide clarity for healthcare facilities that generate these materials.
Understanding how this rule applies to daily operations is essential for maintaining compliance and protecting both public health and the environment.
This rule applies primarily to healthcare facilities that generate hazardous pharmaceutical waste, regardless of whether they operate as very small, small, or large quantity generators. While tiny quantity generators may have flexibility in whether they follow the rule, many choose to adopt it to maintain consistency, reduce risk, and align with best practices.

Understanding Generator Status Under the New Rule
One of the most significant changes introduced by the hazardous pharmaceutical waste rule relates to how generator status is calculated. Under the updated requirements, dangerous pharmaceutical waste, including items classified as P-listed, no longer counts toward a facility’s hazardous waste generator status.
This change eliminates a significant compliance burden for healthcare organizations that frequently handle pharmaceuticals.
Generator status is now determined solely by non-pharmaceutical hazardous waste, including laboratory solvents, cleaning chemicals, and maintenance-related dangerous materials. Facilities must still conduct proper waste determinations for these materials and retain all testing documentation and waste characterization records for a minimum of three years.
Accurate recordkeeping remains a foundational requirement and is frequently reviewed during regulatory inspections.
Prohibition on Flushing Hazardous Pharmaceuticals
A key objective of the new rule is to prevent environmental contamination resulting from improper disposal practices. Flushing hazardous pharmaceuticals down sinks or toilets has been linked to water system contamination and ecological harm.
As a result, the rule establishes a complete ban on flushing dangerous pharmaceutical waste, regardless of generator category.
This prohibition applies strictly to hazardous pharmaceuticals.
Non-hazardous pharmaceutical waste is not covered under this ban, and controlled substances continue to fall under separate regulatory frameworks governed by drug enforcement authorities. Healthcare organizations must clearly distinguish between these waste streams to avoid violations and environmental impact.
Differentiating Between Creditable and Non-Creditable Waste
The rule introduces two distinct categories of hazardous pharmaceutical waste, each with specific handling requirements. Correctly identifying which category applies to a given item is critical for compliance.
Potentially creditable hazardous pharmaceutical waste includes items that may be eligible for manufacturer credit through a reverse distribution process. These items must be in original packaging, undispersed, and either unexpired or within a limited time past expiration. Products subject to recalls are excluded from this category.
Non-creditable hazardous pharmaceutical waste includes items that cannot be returned for credit. This may consist of partially used medications, contaminated pharmaceuticals, expired items beyond their allowable shelf life, or drugs removed from their original packaging.
These materials must be managed as hazardous waste and disposed of in accordance with regulatory requirements.
Container Selection and Storage Requirements
The rule simplifies pharmaceutical waste accumulation by eliminating the traditional satellite and central accumulation area model. Instead, it focuses on container-based requirements that apply consistently across healthcare settings. Facilities may accumulate hazardous pharmaceutical waste for up to one year, provided it is stored in compliant containers.
Containers must be structurally sound, compatible with the waste they hold, and free from rust, leaks, or deterioration. Regular inspections are required to ensure containers remain in good condition and do not pose a risk of release into the environment.
Please address any damaged or leaking containers as soon as possible.
Clear labeling is mandatory and must include the phrase “Hazardous Waste Pharmaceuticals.” Labels must be legible and visible to staff at all times. While the rule allows flexibility in storage location design, facilities are expected to restrict public access and implement reasonable security measures, such as locked storage areas, to prevent unauthorized handling.
Training and Staff Awareness
Effective compliance depends on well-informed staff.
The regulations require training for any employee who generates, handles, transports, or manages hazardous pharmaceutical waste. Training must cover proper handling procedures, container use, emergency response actions, and spill management protocols.
Training may be delivered through online programs, in-person sessions, or structured staff meetings, provided it adequately addresses regulatory requirements. Documentation of training completion should be maintained and updated regularly to ensure accuracy and completeness.
Ongoing education is critical when regulations change or when new pharmaceutical waste streams are introduced.
Preparing for Compliance Timelines
Although the rule provides a delayed implementation period following publication, organizations should not delay preparation.
Early planning allows facilities to review existing disposal practices, update policies, acquire compliant containers, and train staff without operational disruption.
Facilities should conduct internal audits to identify gaps in waste segregation, labeling, and storage practices.
Updating written procedures and ensuring alignment with regulatory language can significantly reduce inspection risk and improve overall compliance readiness.
Environmental and Operational Benefits
Beyond regulatory compliance, proper hazardous pharmaceutical waste management delivers tangible environmental benefits. Preventing pharmaceuticals from entering water systems protects ecosystems and public drinking water supplies.
Clear segregation and disposal practices also reduce staff exposure risks and improve workplace safety.
Operationally, the rule provides greater clarity and consistency for healthcare facilities. Simplified accumulation standards and defined waste categories reduce confusion, allowing organizations to design more efficient waste management workflows.
Final Considerations
The EPA hazardous waste management rule represents a significant advancement in healthcare environmental compliance.
By redefining generator status calculations, banning the flushing of pharmaceuticals, clarifying waste categories, and emphasizing the importance of container integrity and training, the rule creates a more practical and protective framework for managing pharmaceutical waste.
Healthcare organizations that proactively adopt these requirements position themselves to meet regulatory expectations, protect the environment, and ensure the health and well-being of staff and the community. Ongoing education, documentation, and periodic review remain essential to sustaining compliance as regulations and healthcare practices continue to evolve.

Expert Medical Waste Management: With over 25 years of industry experience, Secure Waste is a trusted local leader in hazardous and biohazardous waste disposal across Maryland, Virginia, and Washington, D.C. Specializing in medical waste management, sharps needle disposal, and biohazard waste removal, the company ensures full compliance with federal, state, and local regulations while prioritizing environmental sustainability.
The company also offers additional services, including secure document shredding and sharps container sales, providing comprehensive solutions for healthcare facilities and businesses. Our cost-effective services help clients maintain regulatory compliance without unexpected costs.
With a commitment to customer satisfaction, Secure Waste offers tailored waste management plans that align with industry best practices. Their team of experts provides reliable, timely, and compliant services, making them the preferred choice for medical waste disposal. For a free waste quote or more information, visit www.securewaste.net