What Is OPIM? Other Potentially Infectious Material Explained By Secure Waste: Expert Solutions
Have you ever wondered what OPIM stands for in the context of healthcare? Occupational Safety and Health (OSAH) OPIM is an acronym for “Other Potentially Infectious Materials,” which encompasses a variety of biological substances that may harbor pathogens capable of causing disease.
This includes bodily fluids such as blood, saliva, vomit, human tissue, cell cultures, and certain laboratory specimens.
Many people often confuse OPIM with terms like medical waste or sharps waste, but they are not synonymous. Medical waste refers to all types of waste generated in a healthcare setting that poses a risk to public health or the environment.
In contrast, sharps waste denotes explicitly items that can puncture or cut, such as needles and blades.
Secure Waste has sought clarity on the meaning and implications of OPIM within the medical waste management space. Is OPIM a well-defined term with clearly established guidelines, or is it merely a fabrication that lacks a concrete framework?
In this article, Secure Waste delves deeply into the concept of OPIM, exploring its definitions, regulations, and the best practices for safe handling and disposal. We’d like to join us as we look at this critical topic and its implications for healthcare safety and compliance.
Secure Waste provides a comprehensive overview of the critical nature of managing potentially infectious materials (OPIMs) in healthcare environments.
Whether during diagnosis, prevention, or treatment, these materials are ubiquitous in every medical setting. OPIM, which stands for Other Potentially Infectious Materials, encompasses a variety of substances that may pose health risks.
While blood is one of the most prevalent biohazardous materials encountered by healthcare professionals, OPIM can include various bodily fluids and other biological materials.
Healthcare workers must understand that infectious or biomedical waste terminology can vary among states. Recognizing the distinctions between hazardous/contagious waste and non-hazardous/non-infectious medical waste is paramount for maintaining a safe and compliant healthcare facility.
According to Secure Waste, generating, handling, storing, and disposing of medical waste demands rigorous precautions to protect staff and patients.
Medical professionals face significant risks of exposure to bloodborne pathogens, which can transmit serious diseases such as Hepatitis B, Hepatitis C, and HIV. Thus, a well-informed approach to waste management is vital in safeguarding health and preventing the spread of infectious materials.
What is the meaning of the acronym OPIM?
OPIM, an abbreviation for “Other Potentially Infectious Materials,” refers to substances with a risk of infection. This term is crucial in the context of regulated medical waste, which encompasses any materials that have been contaminated by blood or other potentially infectious substances.
These materials pose significant health risks and are subject to stringent handling and disposal regulations to prevent spreading contagious diseases. Understanding OPIM is essential for ensuring safety in medical environments where the risk of exposure to harmful pathogens exists.
Examples of OPIM consist of:
- Blood Components and Products: This includes vital components such as platelets, red blood cells, plasma, and serum, all of which play crucial roles in the body’s circulatory and immune systems.
- Used Sharps: Items that pose a risk of injury or infection, including syringes, needles, and lancets, which are utilized in various medical procedures.
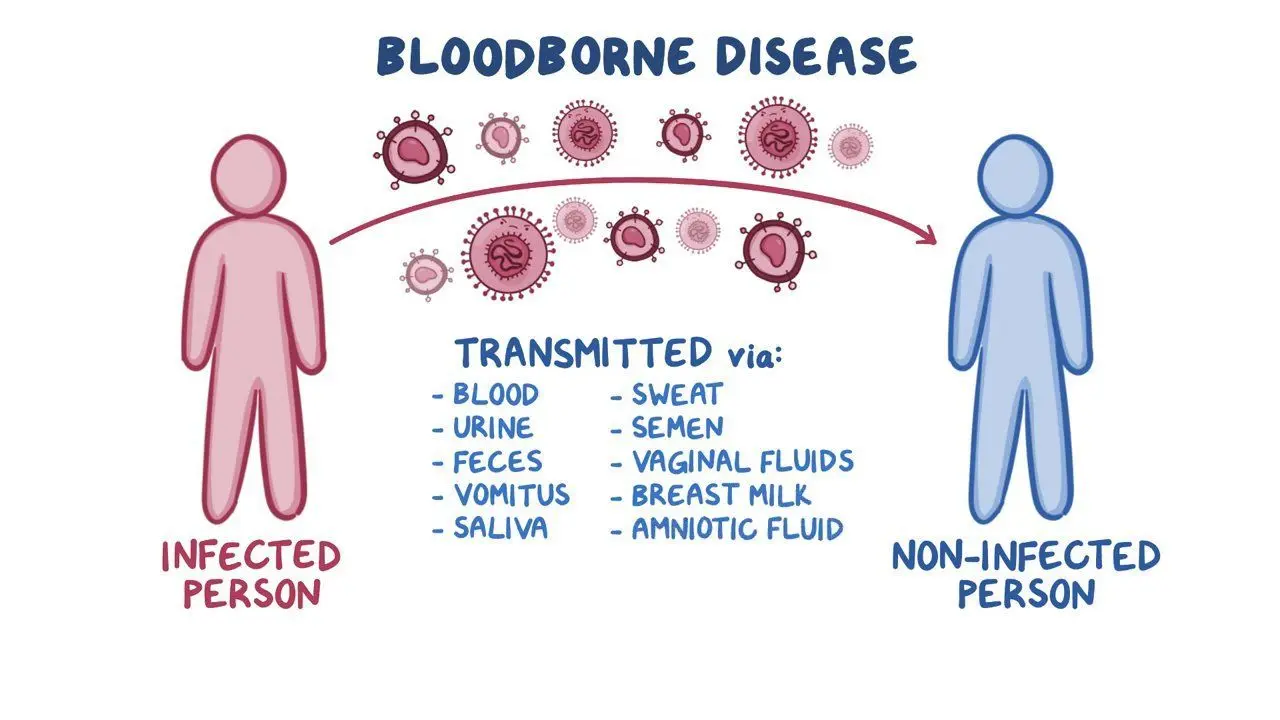
- Body Fluids Contaminated by Blood: This category encompasses a range of bodily fluids that may carry bloodborne pathogens, including vaginal secretions, semen, amniotic fluid, pleural fluid, peritoneal fluid, nasal secretions, cerebrospinal fluid, and urine and feces.
- Pathology Wastes: This is a critical category involving unfixed tissue cultures, organs, or human and animal body parts, which require careful handling and disposal due to potential health hazards.
- Saliva Contaminated with Blood: Saliva that contains visible traces of blood, often resulting from dental procedures, poses a risk of infection transmission.
- Personal Protective Equipment (PPE) Contaminated with Blood: Gear such as gloves, gowns, and masks exposed to blood, necessitating special disposal measures to prevent cross-contamination.
- Other Red Bag Biohazard Wastes: Any additional medical waste designated for biohazard disposal is typically marked with a red bag to indicate its potential risk to human health.
How are bloodborne pathogens transmitted?
When handling biohazardous waste, it is crucial to prioritize safety by ensuring thorough training, employing stringent precautions, and always wearing appropriate personal protective equipment (PPE).
Bloodborne pathogens in human blood and other potentially infectious materials pose significant health risks and can be transmitted in various ways. Here are some common routes of transmission:
- Needle stick injuries: Accidental punctures or cuts from used sharps devices can introduce contaminated blood directly into the bloodstream.
- Contact with broken skin: Infectious agents can come into contact with cuts, abrasions, or open wounds, posing a risk of infection.
- During childbirth, Pathogens can be transmitted from mother to child during labor and delivery.
- Sexual contact: Engaging in unprotected sexual activity can facilitate the transmission of bloodborne pathogens.
- Sharing needles: Reusing or sharing needles for drug use increases the likelihood of spreading infections.
- Other activities involving bodily fluids: Any action involving exchanging human blood or body fluids carries a risk of transmitting these pathogens.
By understanding and recognizing these transmission routes, individuals can take necessary precautions to protect themselves and others from potential infection.
Dive into the crucial information surrounding bloodborne pathogens Understanding these hidden dangers is vital for safety in various environments From recognizing the risks to implementing effective prevention strategies this guide will equip you with the knowledge you need to protect yourself and others Lets explore the key aspects together
Dangers of Spreading Bloodborne Pathogens through Other Potentially Infectious Materials (OPIMs)
Bloodborne pathogens pose significant health risks, with some of the most prevalent being Hepatitis B, Hepatitis C, and HIV (human immunodeficiency virus).
Healthcare personnel experience approximately 385,000 sharps injuries annually, highlighting the importance of treating human blood components and other potentially infectious materials with the utmost caution and respect.
OSHA sets the Bloodborne Pathogens Standards
The Occupational Safety and Health Administration (OSHA) is crucial in safeguarding workplace health by enforcing comprehensive safety standards and promoting educational initiatives.
The OSHA standard for bloodborne pathogens is particularly significant among its various regulations. It mandates that employers take proactive measures to protect their employees from potential hazards related to occupational exposure to blood and other potentially infectious materials (OPIMs). This standard ensures that workers have the knowledge and resources they need to maintain safe practices, thereby minimizing the risk of exposure and fostering a safer work environment.
OSHA’s standards for bloodborne pathogens consist of
- Exposure control plan
Consider this a comprehensive written strategy outlining how your facility will effectively eliminate or significantly reduce the risks associated with anticipated exposure to hazardous materials. The plan should include a detailed examination of the potential hazards that workers may encounter, emphasizing the importance of identifying specific risks related to bloodborne pathogens.
An essential aspect of this plan is the commitment to an annual review, which should evaluate and incorporate technological advancements to mitigate exposure risk further. Additionally, the documentation must highlight safer devices identified and implemented to protect employees.
Input from employees who are directly involved in patient care is crucial; their firsthand experiences and suggestions can provide valuable insights into improving safety protocols.
Furthermore, this comprehensive safety plan should be readily available for inspection by OSHA or other regulatory bodies upon request.
Lastly, compiling a thorough list of all job roles and tasks that may expose employees to bloodborne pathogens is essential, ensuring that all relevant risks are recognized and addressed.
- Controls for work practices
Strategies for minimizing exposure to bloodborne pathogens involve implementing specific safety protocols that can significantly lower the risk. For instance, training employees on the proper technique for safely capping needles—such as the one-handed scoop method—can dramatically reduce the likelihood of needle-stick injuries.
Additionally, employing a color-coded system for medical waste containers enhances organization. It ensures employees can easily identify and dispose of hazardous materials, safeguarding their health and well-being. These measures, ongoing education, and vigilance are crucial in creating a safer work environment.
- Necessary vaccinations and subsequent check-ups
Employers must provide the hepatitis B vaccine to all employees with potential exposure due to their job responsibilities, ensuring it is available at no cost.
This vaccination must be offered within 10 working days of the employee’s initial exposure to tasks that present this risk, unless the individual has already received the vaccine, has undergone antibody testing confirming immunity, or cannot accept the vaccine due to valid medical reasons.
- Interaction and yearly training.
Training and certifications play a crucial role in ensuring workplace safety and compliance. Bloodborne pathogen training should be conducted immediately when there is a risk of exposure, and employees must participate in refresher courses at least once a year thereafter.
Furthermore, depending on the introduction of new tasks or procedures in the workplace, additional training may be necessary to equip staff with the knowledge and skills required to navigate these changes safely and effectively.
- Required record keeping
Maintaining a comprehensive and meticulously documented record for every employee with occupational exposure is essential. This record should include the employee’s full name, a copy of their vaccination status, and relevant dates or documented reasons for any medical exemptions.
Additionally, it must contain copies of all examination results, follow-up assessments, and a written opinion from a qualified healthcare professional regarding their health status. Furthermore, a detailed account of all training sessions related to workplace safety and health practices should be included.
All records must be systematically organized, securely stored, and readily accessible for review by OSHA or other relevant regulatory authorities when requested.
In todays world ensuring safety in our environments is more crucial than ever especially when managing exposure to occupationally acquired infections Lets dive into the fascinating yet vital topic of Other Potentially Infectious Materials OPIM and bloodborne pathogens exploring the essential practices that keep us safe and healthy in various settings With the proper knowledge and precautions we can significantly reduce risks and protect ourselves and those around us
OPIM: Safeguarding Against Potentially Infectious Materials
Summary of potentially infectious materials
To keep everyone safe, it’s crucial to implement effective handling, segregation, storage, packaging, and disposal procedures for potentially infectious materials (OPIM). Although OSHA doesn’t directly regulate medical waste disposal, its guidelines promote safety and compliance throughout the healthcare industry.
Healthcare facilities and institutions that produce OPIM should enthusiastically consult federal, state, and local regulations to ensure a smooth and effective waste disposal process.
OSHA’s Blood-borne Pathogen Standard (29 CFR 1910.1030) provides essential protections for employees handling regulated waste, ensuring a safer environment from potential spillage or exposure to OPIM. By following these practices, we can collectively contribute to a healthier future!
OPIM or Other Potentially Infectious Materials refers to specific categories of biological materials that may pose a risk of infection This term typically encompasses items such as blood certain body fluids and other materials that may harbor pathogens In healthcare and waste management its crucial to determine whether OPIM qualifies as medical waste Medical waste is generally defined as any waste that is generated during the diagnosis treatment or immunization of humans or animals which can potentially pose a health risk to the community Understanding the classification of OPIM is essential for proper management and disposal to ensure safety and compliance with health regulations
Instructions for OPIM Disposal AKA Medical Waste
At Secure Waste, we emphasize the importance of precise terminology in effectively managing medical waste within various healthcare settings. Navigating the complexities of compliance regulations can be challenging, especially when interpretations of specific terms can vary among individuals.
For instance, OSHA’s “contaminated” and “potentially contaminated” classifications are subjective and depend on practical application. It’s essential to recognize that not all items labeled as “potentially contaminated” qualify as regulated medical waste.
To effectively address these challenges, we advise healthcare professionals involved in biomedical waste management to take a conservative approach to waste classification. This can include implementing color-coded containers, dedicated waste bins, and specialized sharps containers strategically positioned throughout the facility.
Comprehensive training for all staff members—from management to housekeeping—is crucial to understanding the different waste streams and the appropriate disposal methods for other potentially infectious materials (OPIM).
In some cases, the individual managing the waste must assess whether an item contains small or visible amounts of blood. However, the subjective nature of what constitutes “small” can lead to confusion. Generally, items believed to contain minimal OPIM or slight traces of blood may be disposed of in standard plastic-lined trash bins. Still, this decision should be made with careful consideration.
By adopting these best practices, healthcare facilities not only comply with regulatory standards but also prioritize the health and safety of everyone in these environments.
In conclusion
Now that you have a more comprehensive understanding of OPIM waste disposal, don’t hesitate to contact Secure Waste.
We provide reliable, compliant, eco-friendly medical waste disposal solutions for your facility’s needs. We have expertise in biomedical, hazardous waste, and Sharps container disposal. In addition, we provide customized waste management plans, including secure collection and transport, and sustainable disposal practices.
Contact us today for a FREE Waste Assessment, or request a quote online!
**Disclaimer** This information is provided for reference purposes only and should not be considered as legal advice or factual information at the time of your reading. Regulations frequently change and can vary from state to state. We encourage you to contact your local regulatory authorities or Secure Waste directly for the most current information. Please note that Secure Waste is not liable, in part or in whole, for any information contained on this page or website.

Expert Medical Waste Management: With over 25 years of industry experience, Secure Waste is a trusted local leader in hazardous and biohazardous waste disposal across Maryland, Virginia, and Washington, D.C. Specializing in medical waste management, sharps needle disposal, and biohazard waste removal, the company ensures full compliance with federal, state, and local regulations while prioritizing environmental sustainability.
The company also offers additional services, including secure document shredding and sharps container sales, providing comprehensive solutions for healthcare facilities and businesses. Our cost-effective services help clients maintain regulatory compliance without unexpected costs.
With a commitment to customer satisfaction, Secure Waste offers tailored waste management plans that align with industry best practices. Their team of experts provides reliable, timely, and compliant services, making them the preferred choice for medical waste disposal. For a free waste quote or more information, visit www.securewaste.net