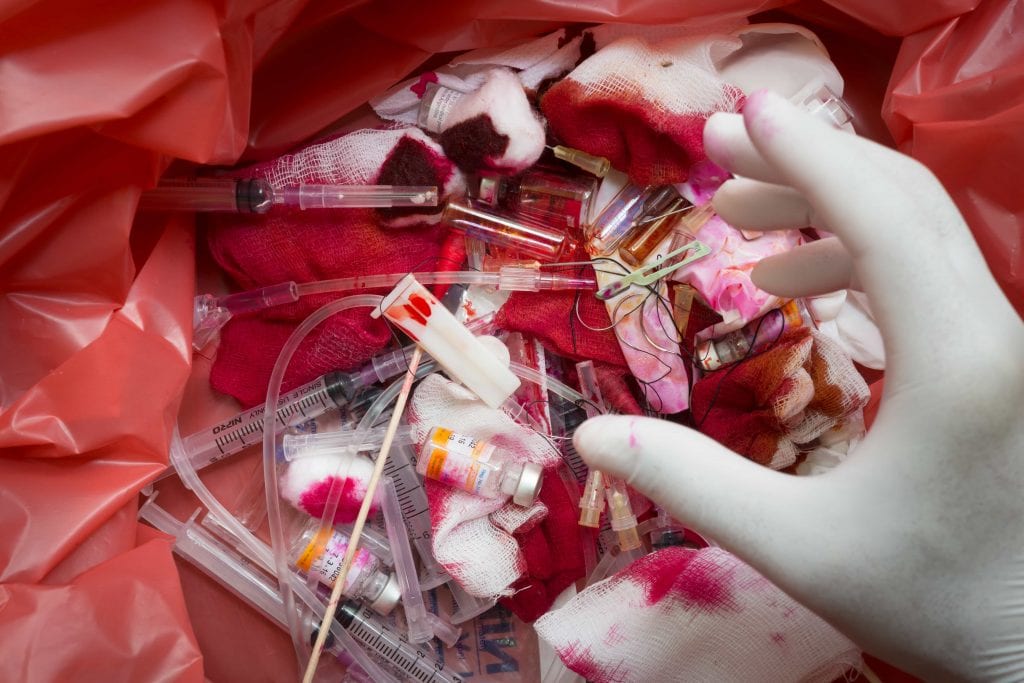
Regulated Medical Waste: The Ultimate Guide From Secure Waste Expert Solutions And A Deep Dive Into What Is Medical Waste
Are you a healthcare provider seeking an authoritative and comprehensive guide on medical waste?
Whether you are a physician, dentist, clinic operator, surgery center staff, funeral director, tattoo artist, or any professional handling daily biohazardous materials and sharps waste, we have you covered. Have you ever wondered why it’s termed “medical waste”?
Or are you curious about the proper disposal methods and the regulatory bodies overseeing this crucial aspect of healthcare? Look no further! Secure Waste provides an in-depth exploration and the ultimate resource for everything you need to know about medical waste management.
We’d like you to join us as we delve into the intricacies of this vital subject today.
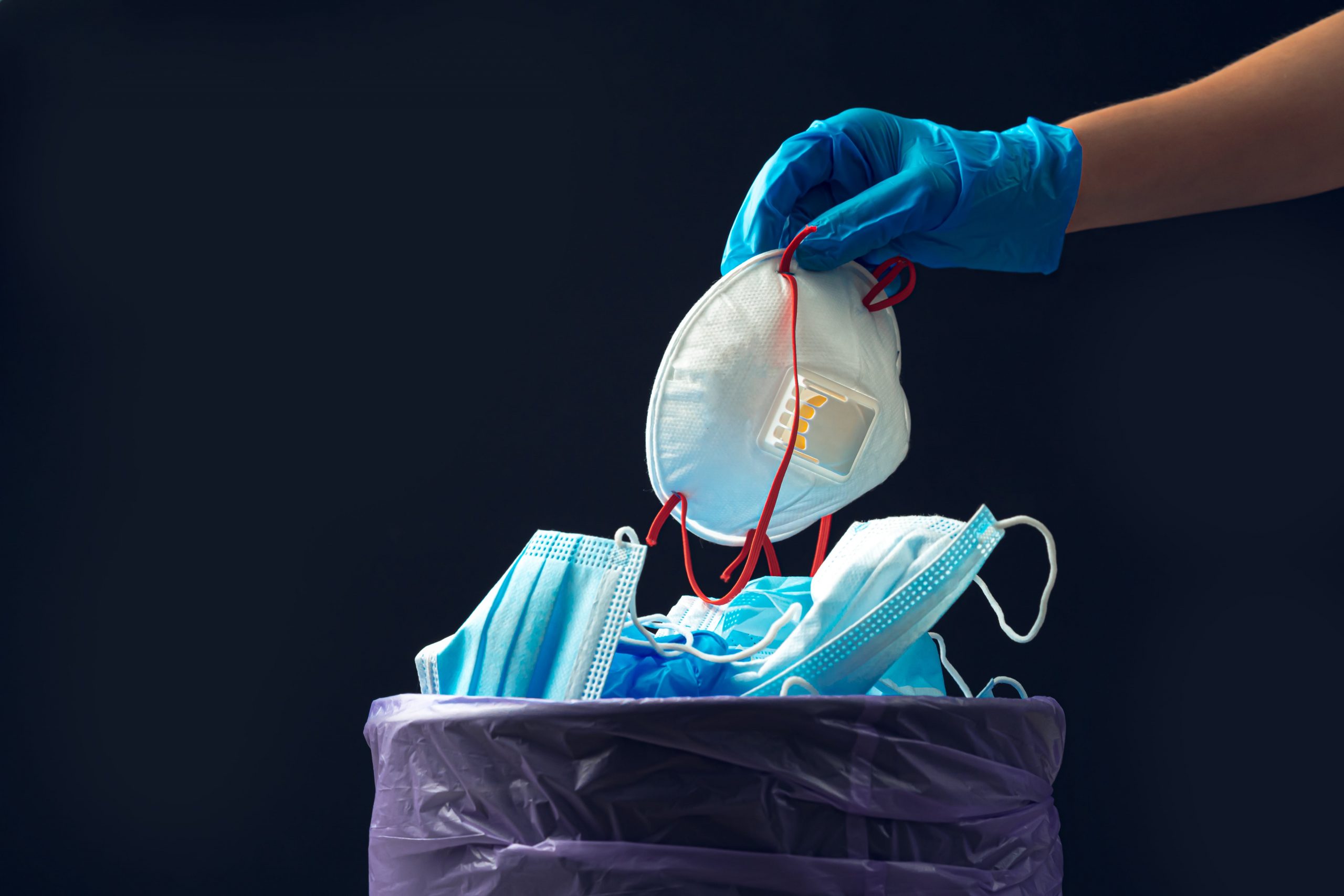
Medical Waste: The A To Z And Everything In Between
Let’s Get Started: What Is Medical Waste? The Ultimate Definition
Medical waste, commonly known as healthcare medical waste or biomedical waste, refers to a wide array of discarded materials generated while providing healthcare services.
This waste often poses significant risks as it can harbor infectious agents, thereby making its effective management paramount for safeguarding public health and safety. Common examples of medical waste include used needles, blood-stained bandages, and other items that may have come into contact with infectious materials.
Moreover, anatomical waste—human or animal body parts—further emphasizes the necessity for meticulous disposal practices that adhere to stringent health standards.
Types of Medical Waste Explained In Detail:
- Regulated Medical Waste (RMW) or Red Bag Waste: This category includes items heavily contaminated with blood or other potentially infectious materials (OPIM).
Typical examples are blood-soaked bandages, gauze, and various forms of personal protective equipment (PPE), all of which must be handled with utmost care to mitigate any risk of infection and ensure safe disposal.
- Sharps Medical Waste: This segment encompasses items designed to penetrate or incise skin, such as needles, syringes, scalpel blades, and surgical instruments.
Due to their hazardous nature, sharps must be disposed of in specialized containers that prevent accidental injuries and ensure safe handling.
- Anatomical Medical Waste: This type includes human or animal body parts, tissues, and organs, necessitating careful management to uphold ethical considerations and protect community health.
The disposal of such materials must align with established protocols to honor the deceased’s dignity and prevent potential biological risks.
- Pathological Medical Waste: This category comprises human fluids, body tissues, blood, and other biological materials generated during medical procedures.
Such waste requires diligent handling to avoid contamination and the spread of infection, highlighting the importance of proper disposal methods.
- Laboratory Medical Waste: This refers to cultures and stocks of infectious agents, alongside contaminated materials such as petri dishes, glassware, and other laboratory instruments that pose significant biological hazards.
Safe disposal of laboratory waste is essential to prevent accidental exposure to pathogens.
- Pharmaceutical Waste: This includes expired, unused, or unwanted medications that require specific disposal methods to mitigate potential harm to human health and the environment.
Proper pharmaceutical waste management is crucial to prevent drug misuse and environmental contamination.
Ensuring the proper disposal of medical waste is vital for preventing infection transmission, protecting public health, and maintaining environmental integrity.
The complexities of medical waste management underscore the need for stringent adherence to regulations and best practices within healthcare facilities.
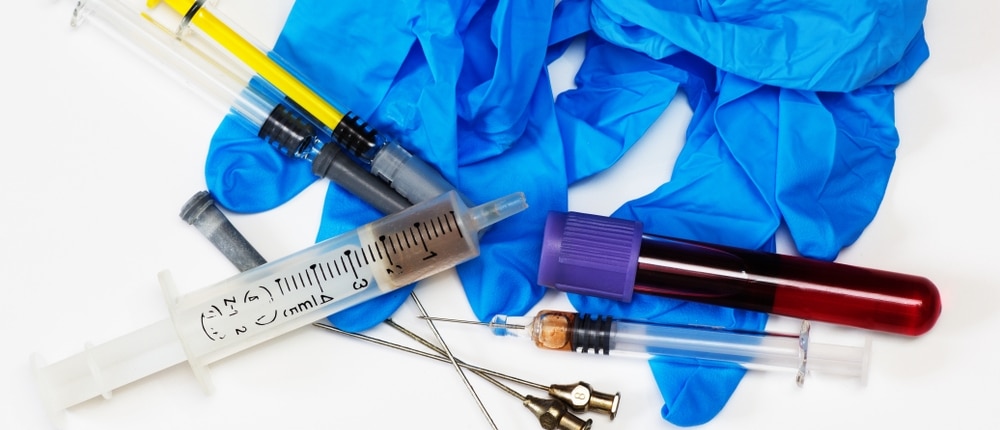
Why Call It Medical Waste?
Medical waste is commonly used in healthcare to refer to any waste generated from medical activities.
However, this type of waste can be known by various names, each reflecting different aspects of its origins and management.
The primary term is “regulated medical waste,” but it can also be referred to as “biohazard waste,” “biomedical waste,” or simply “biohazardous waste.”
In some contexts, you might encounter terms like “red bag waste,” which indicates the specific type of bags used for disposal, or “sharps waste,” which pertains to items that can puncture or cut, such as needles and blades.
It is essential to distinguish between medical and hazardous waste, as they are not the same and should not be mixed.
In summary, while “medical waste” is the overarching term, it encompasses several other names, each with significance within the healthcare waste management framework.

Now That You Know Why We Call It Medical Waste, Let’s Discuss Who Regulates It
Medical Waste Tracking Act of 1988
After discovering medical waste on several East Coast beaches, concerns about potential health risks led Congress to enact the Medical Waste Tracking Act (MWTA) in 1988. This legislation, which amended the Solid Waste Disposal Act, introduced several key provisions:
- Definition of Medical Waste: The act defined medical waste and outlined which types would fall under its regulatory framework.
- Tracking System: It established a cradle-to-grave tracking system that requires medical waste generators to initiate tracking through a designated form.
- Management Standards: The MWTA established management standards for medical waste, including segregation, packaging, labeling, marking, and storage.
- Record Keeping and Penalties: It imposed record-keeping requirements and specified penalties for mismanagement of medical waste.
The Environmental Protection Agency (EPA) implemented the MWTA regulations on March 24, 1989.
These regulations governed a two-year program that commenced on June 24, 1989, and concluded on June 21, 1991, operating in New York, New Jersey, Connecticut, Rhode Island, and Puerto Rico. During this timeframe, the EPA gathered valuable information and conducted multiple studies related to medical waste management. The MWTA and the EPA’s program raised awareness about medical waste issues.
They served as a framework for states and other federal agencies to develop medical waste management programs.
Moreover, the MWTA mandated that the EPA evaluate various treatment technologies available to determine their efficacy in reducing the disease-causing potential of medical waste. In 1990, the technologies assessed included:
- Incinerators and autoclaves (both on-site and off-site)
- Microwave units
- Various chemical and mechanical systems
The data collected under the MWTA and concurrent EPA investigations concluded that the risk of disease caused by exposure to medical waste is highest at the point of generation and diminishes thereafter.
Consequently, the risk posed to the general public is significantly lower than that faced by individuals occupationally exposed to medical waste.

Understanding the life cycle of medical waste is crucial for ensuring safety and sustainability Join us on an informative journey to uncover medical wastes path from its initial generation in healthcare settings to its final disposal This process involves various stages designed to manage waste efficiently while protecting public health and the environment Lets explore how proper handling and treatment of medical waste can significantly impact our communities and beyond
What Is The Medical Waste Cycle
Effective medical waste management encompasses a comprehensive process beyond the point where waste is removed from a facility.
Gaining insight into each stage of the waste cycle, including the procedures after materials leave your premises, is essential for ensuring that your waste management practices are compliant and environmentally sustainable. Below is a concise explanation of each medical waste management continuum stage.
- Generating Waste: In the dynamic healthcare delivery environment, healthcare workers can generate a wide array of medical waste during patient care.
- Regulated Medical Waste (RMW): This category comprises waste that poses a potential risk of infection and is subject to stringent regulations governing its collection, transportation, treatment, and disposal. Items classified as biohazardous waste, particularly those saturated with blood or other potentially infectious materials, are essential within this realm. This includes soiled gauzes, patient drapes, and specific types of personal protective equipment that can harbor pathogens.
- Sharps: A critical subset of RMW, sharps are defined as any object capable of puncturing the skin. This includes an extensive range of potentially dangerous items such as needles, scalpels, syringes, and even fragmented glass, all of which can pose significant health risks if not managed properly.
- Unused or Expired Pharmaceuticals: This waste stream encompasses a variety of medications that may be deemed non-hazardous, alongside more dangerous substances like warfarin. The improper disposal of these pharmaceuticals can lead to environmental contamination and public health concerns.
- Hazardous Chemicals: This category includes a variety of toxic substances, such as cleaning fluids and other dangerous chemicals that can pose severe health risks to humans and the environment if mishandled.
- Chemotherapeutic Waste: Any waste generated from the administration of chemotherapy falls within this category. This hazardous waste requires specialized handling due to its potential to cause harm to healthcare workers and the public.
- Controlled Substances: These include any drugs regulated by the Drug Enforcement Administration (DEA), such as opioids. Due to their potential for abuse and addiction, these substances require meticulous care in both usage and disposal.
Various regulatory bodies oversee the management and disposal of medical waste, each tasked with ensuring safety and compliance. For instance, the Occupational Safety and Health Administration (OSHA) oversees the safe handling of waste containing bloodborne pathogens, encompassing sharps and other regulated medical waste.
Meanwhile, individual state agencies establish regulations to dispose of RMW, accounting for state-specific public health needs.
The Environmental Protection Agency (EPA) regulates the disposal of hazardous waste pharmaceuticals, ensuring that these substances do not pollute air and water resources.
Additionally, the Drug Enforcement Administration (DEA) oversees the handling and disposal of controlled substances, providing strict guidelines to mitigate the risks associated with these powerful drugs. Once medical waste is removed from the healthcare facility, the Department of Transportation (DOT) governs its transportation, enforcing regulations that ensure hazardous materials are transported safely and responsibly.
- Segmenting Waste: It is essential for healthcare professionals to meticulously segment waste to ensure the safety of healthcare workers, minimize the spread of harmful pathogens, and facilitate appropriate treatment after waste exits the facility. This involves disposing of waste in clearly marked containers specifically engineered to hold each type of waste securely.
For example, most regulated medical waste (RMW), except sharps and chemotherapeutic waste, can be efficiently disposed of in hygienic red bags designed to fit seamlessly within designated waste containers.
Regarding sharps disposal, healthcare staff must utilize FDA-cleared, puncture-proof containers that safeguard against potential injuries.
These containers can be reused, significantly reducing plastic waste that ultimately ends up in landfills.
Moreover, the healthcare system offers specially designed containers tailored for various types of waste, including controlled substances, pharmaceuticals, hazardous chemical waste, and trace amounts of chemotherapeutic waste, ensuring that each category is handled with the utmost care and in compliance with regulations.
- Preparing Waste Containers for Transport: When a waste receptacle reaches capacity, designated staff must meticulously prepare it for transport to the waste management facility.
This preparation process involves securely sealing the container’s contents to prevent potential leakage that could compromise safety.
For red bags containing non-sharp Regulated Medical Waste (RMW), the closed bag should be carefully placed within a sturdy transport container.
Once the outer container is securely closed and sealed, no part of the bags must be visible, ensuring compliance with safety standards and promoting prudent waste management practices.
Suppose the waste is scheduled to be picked up by a licensed waste hauler.
In that case, it is vital to adhere to Department of Transportation (DOT) guidelines regarding proper marking, the weight of the box, manifest or shipping paper details, and any other applicable regulations. In cases where the waste is being mailed to a waste management facility, strict compliance with postal requirements is necessary.
Please use appropriate labels that meet the standards the United States Postal Service (USPS) set forth to ensure safe and legal waste transport.
- Stage Four: Treating Waste: Upon arrival at the waste management facility, a dedicated medical waste management partner carefully orchestrates the handling of medical waste, ensuring adherence to the facility’s specific permit requirements, as the relevant state regulatory agency dictates.
The predominant method for treating medical waste is autoclaves, sophisticated machines designed to transform hazardous materials into non-infectious waste. Initially, trained staff meticulously weigh the incoming waste before placing it into designated bins, which are then transported to the autoclave.
Within this controlled environment, air is effectively evacuated and replaced with high-pressure, high-temperature steam, maintained for a critical period of 20 to 30 minutes. This intense process is highly effective in eradicating any harmful pathogens that may be present within the waste. Once the cycle is complete, the “cleaned” waste is compacted, making it ready for safe transport.
Incineration is the required treatment method in certain instances, particularly for materials such as sharps contaminated with pathological or trace chemotherapeutic waste.
This process applies extreme heat to the waste, facilitating thorough combustion that eliminates any hazardous elements, reducing them to ash. This method is crucial, destroying the waste and minimizing the risk of harmful chemicals seeping into local waterways.
Your medical waste management partner will provide detailed shipping information to maintain transparency and regulatory compliance. This documentation serves as evidence of compliance and can be pivotal should any regulatory authority require verification of the waste treatment process.
- Sending Treated Waste to Its Final Destination: Once medical waste has been treated to be rendered non-infectious or destroyed, it embarks on its journey to a final disposal site.
Depending on local regulations and practices, this site may be a landfill or a waste-to-energy (WTE) facility. The transformation of waste directed to a WTE site is remarkable: converted into valuable, clean electricity.
This sophisticated WTE process employs specially designed boilers that ignite non-hazardous waste within a closed-loop system, ensuring minimal environmental impact.
As the materials combust, they release an immense amount of heat, which is captured and repurposed to produce steam. This steam then drives a turbine, generating electricity dispatched to local utility companies and ready to power homes and businesses.
Alternatively, this generated electricity can be returned to the waste management facility, providing energy to operate equipment and help sustain the waste processing operations, creating a circular and efficient system.

Ensuring the safe packaging of medical waste is crucial for health and safety Follow this guide for clear and practical steps to get it done right
How To Package Your Medical Waste For Disposal
To effectively package medical waste, choosing an appropriate container, either a single-use cardboard box or a robust reusable container, is essential.
You can start by lining the chosen container with a red biohazard bag, making sure that the bag extends over the sides of the container for added security. Carefully place the regulated medical waste inside the bag, diligently sealing the bag and the container securely.
Lastly, could you mark the container with a prominent biohazard symbol and include any additional required labeling for clear identification?
Here’s a more detailed breakdown of the process:
- Selecting the Right Container:
- Single-use boxes: Opt for sturdy corrugated cardboard boxes, which may require reinforcement at the bottom with tape to ensure stability and prevent spillage.
- Reusable containers: Ensure they are leak-proof and feature a secure locking mechanism, such as an auto-locking lid. Inspecting them for cracks or damage before every use is crucial to maintain safety.
- Lining the Container:
- Line the container’s interior with a vivid red biohazard bag, ensuring it adequately overlaps the edges of the container. Adhere to state-specific regulations concerning the thickness of the bag and the maximum weight allowable.
- Placing Waste Inside:
- Carefully deposit only regulated medical waste into the lined bag. Ensure that the bag is not overfilled—ideally, around 2/3 complete—for proper closure. Use designated puncture-resistant containers to ensure safety for items that can cause injury, such as needles or scalpels.
- Sealing the Bag and Container:
- Bag: To secure the bag, twist the top to form a “gooseneck” and fasten it with tape or a zip tie, avoiding knots that could lead to leaks.
- Container: Close the container using the auto-locking mechanism, tape, or another suitable method to guarantee a tight seal.
- Labeling:
- Clearly label the container with a significant, easily recognizable biohazard symbol alongside the words “Biohazardous Waste” or “BIOHAZARD.” Additionally, include an emergency contact name and phone number for quick reference in case of an incident.
- Storage:
- Store the medical waste in a designated area that is adequately ventilated, pest-free, and inaccessible to unauthorized individuals. Ensure that the containers are kept closed tightly and safeguarded from environmental elements.
Important Considerations:
- State Regulations: Always consult and adhere to your state’s specific regulations governing the packaging and disposal of medical waste to ensure compliance.
- Proper Closure: Verify that all containers are sealed appropriately to prevent leaks and potential contamination.
- Segregation: To uphold safety standards, medical waste must be kept strictly separated from other types of waste, such as medications, general trash, or recycling.
By following these meticulous steps, you can ensure the safe and responsible handling of medical waste, protecting public health and the environment.
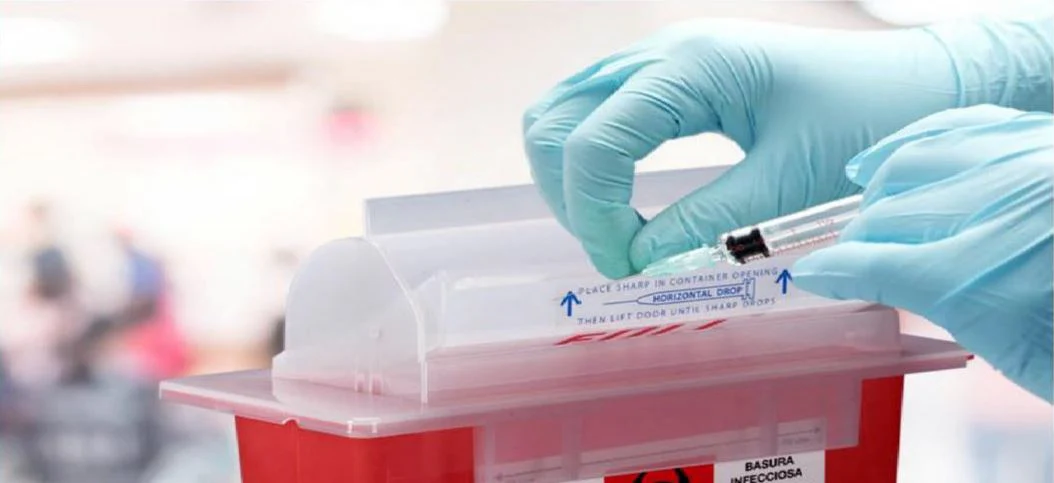
How To Choose A Sharps Container
A sharps container is a specialized receptacle engineered with precision and care to safely dispose of medical needles, syringes, lancets, and other sharp medical instruments. These vital containers protect healthcare professionals and the general public from accidental injuries and significantly reduce the risk of dangerous infections.
Key Features of Sharps Containers:
- Puncture-resistant Construction: Crafted from robust, high-quality plastic or durable metal, these containers are meticulously designed to withstand the penetration of needles and other sharp objects, maintaining their integrity and preventing breaches.
- Leak-resistant Design: With a carefully engineered structure that prevents spills and leaks of potentially infectious materials, sharps containers effectively contain hazardous waste, safeguarding people and the environment.
- Secure, Tight-fitting Lid: The lids are expertly designed to provide a firm closure that prevents accidental exposure to the hazardous contents and enhances safety during transport and handling.
- Prominent Labeling: Each container is typically adorned with a universally recognized biohazard symbol alongside clear and bold labeling that reads “Sharps,” instantly signaling the hazardous nature of the contents.
- Defined Fill Line: A marked fill line inside each container serves as a crucial guide, alerting users when the container is nearing capacity and needs to be responsibly disposed of.
Why Are They Important?
Sharps containers play an indispensable role in the prevention of needlestick injuries, which pose a significant threat in the transmission of bloodborne pathogens such as HIV and Hepatitis. By enabling the safe disposal of contaminated sharps, these containers help inhibit the spread of infections, ultimately protecting healthcare workers and the wider community.
What Can Go in a Sharps Container?
- Needles and syringes
- Lancets
- Auto-injectors
- Infusion sets
- Broken glass from medical devices
- Blunted needles
- Other sharp instruments
What Should NOT Go in a Sharps Container?
- Medical waste like bandages, gauze, and paper
- Empty vials
- Uncontaminated broken glass
- Other types of non-sharp medical waste
Where to Find Sharps Containers:
Sharps containers are readily available at various locations, making it easy to find a suitable option, including:
- Local pharmacies
- Medical supply companies
- Healthcare facilities
- Online retailers
A sturdy household plastic container may be used for temporary disposal solutions; however, it must be replaced with an FDA-approved container as soon as possible.
Important Considerations:
- Always follow local regulations regarding sharps disposal to ensure compliance and safety for everyone involved.
- Never recap, bend, or break needles before disposal, as these practices can increase the risk of injury.
- Promptly dispose of sharps containers when they are three-quarters full to adhere to safety standards.
- Ensure that sharps containers are stored out of reach of children and pets, reducing the risk of accidental injuries and safeguarding the well-being of all.
Expand Your Knowledge With Medical Waste: Other Need To Know By Secure Waste

Expert Medical Waste Management: With over 25 years of industry experience, Secure Waste is a trusted local leader in hazardous and biohazardous waste disposal across Maryland, Virginia, and Washington, D.C. Specializing in medical waste management, sharps needle disposal, and biohazard waste removal, the company ensures full compliance with federal, state, and local regulations while prioritizing environmental sustainability.
The company also offers additional services, including secure document shredding and sharps container sales, providing comprehensive solutions for healthcare facilities and businesses. Our cost-effective services help clients maintain regulatory compliance without unexpected costs.
With a commitment to customer satisfaction, Secure Waste offers tailored waste management plans that align with industry best practices. Their team of experts provides reliable, timely, and compliant services, making them the preferred choice for medical waste disposal. For a free waste quote or more information, visit www.securewaste.net