Medical Waste Disposal: Stopping Drug Diversion
Secure Waste frequently receives inquiries about drug diversion in the healthcare industry. We are a leading provider of healthcare waste management services in Maryland, Virginia, and Washington, D.C.
Our comprehensive services include pharmaceutical disposal, biomedical waste management, sharps container disposal, and specialized training for healthcare waste, drug diversion, and prevention.
At Secure Waste Receivers, we recognize the crucial importance of proper waste disposal in maintaining the integrity of healthcare systems and ensuring patient safety. Our team of experts is committed to ensuring compliance with all applicable local, state, and federal regulations governing hazardous waste management.
We also offer customized training programs designed to educate healthcare personnel on recognizing and preventing drug diversion, fostering a culture of safety, and promoting best practices in waste disposal.
For more information about our services or to discuss how we can assist your healthcare facility, please get in touch with us today at 877-633-7328. Your commitment to safe and responsible waste management is our top priority.

Drug diversion refers to the illegal transfer of prescription medications from legal intended use to illicit use In the healthcare sector this is a significant concern as it can undermine patient safety lead to substance abuse and create legal liabilities for healthcare providers Effective monitoring and regulatory measures are essential to prevent drug diversion ensuring that medications are used appropriately and safely within clinical settings It is crucial for healthcare professionals to be aware of the signs of diversion and to implement best practices to minimize risks ultimately safeguarding both patients and the integrity of the healthcare system
Safety Stopping Drug Diversion
Preventing drug diversion and ensuring proper disposal of controlled substances are critical challenges for patient and staff safety in modern healthcare environments.
Drug diversion, the illegal misappropriation of medications intended for patients, poses serious risks ranging from compromised patient care to staff addiction, regulatory violations, and environmental contamination.
Effective medical waste disposal protocols protect healthcare workers and patients while supporting OSHA and HIPAA compliance requirements that govern every aspect of healthcare operations.
This comprehensive guide outlines expert strategies for secure waste removal and controlled substance management in healthcare settings across all care levels, from large hospital systems to small clinics and long-term care facilities.
By following evidence-based best practices and adhering to strict regulatory guidelines, facilities can enhance their healthcare waste management programs, close security gaps that facilitate diversion, and promote sustainable, compliant practices that protect communities and the environment.
Understanding Drug Diversion in Healthcare Settings
Drug diversion occurs when controlled medications, especially opioids and other painkillers, benzodiazepines, stimulants, and other scheduled substances, are stolen or mishandled by healthcare workers, visitors, patients, or others with access to healthcare facilities.
The ongoing opioid crisis has made this a high-stakes issue with devastating consequences for individuals and communities. Controlled substance use in hospitals is rising to meet legitimate pain management needs, and experts report a corresponding increase in diversion incidents that threaten patient safety and worker wellbeing.
In fact, a 2025 Wolters Kluwer report found nearly all healthcare leaders believe diversion is occurring in U.S. hospitals at some level, yet about two-thirds are not confident their prevention programs are effective at detecting and stopping these incidents.
This gap in confidence underscores the urgent need for stronger safeguards, better technology solutions, and more comprehensive oversight mechanisms. The disconnect between awareness and confidence reveals systemic vulnerabilities that diverters can exploit.
Notably, recent industry data show hospitals are ramping up their response to this growing threat.
One report revealed a 61% increase in diversion investigations from 2023 to 2024, as facilities adopted advanced analytics technology, established formal oversight committees, and implemented stricter protocols to monitor drug handling from the pharmacy to the bedside and through disposal.
These proactive actions have helped reduce discrepancies in an encouraging trend. Only about 6% of controlled substance transactions showed variances in 2024, down from higher rates in previous years, indicating that tighter controls, improved documentation systems, and enhanced surveillance are having a measurable impact on preventing diversion.
Diversion prevention matters on multiple levels, extending beyond the obvious concerns about drug theft. It protects patients from receiving unexpected doses, diluted medications that provide inadequate pain relief, or contaminated medications that have been tampered with by diverters.
It stops staff members from developing or continuing substance abuse disorders that compromise their judgment, performance, and well-being. It also prevents serious environmental harm that extends beyond hospital walls.
Inappropriate disposal methods, such as flushing drugs down toilets or tossing them into regular trash, can pollute water supplies with pharmaceuticals that harm aquatic ecosystems, enable illicit use by individuals who scavenge waste, and create public health hazards.
Specialists note that dedicated one-way disposal containers explicitly designed for sharps and unused medications keep controlled substances out of the water system, unlike flushing or dumping practices that were once common but are now prohibited.
In most modern hospitals, controlled substances are now treated as a formal, regulated waste stream requiring specialized handling, tracking, and destruction methods. Some experts anticipate that mandatory controlled substance disposal programs will become federal requirements in the future, rendering proactive implementation a strategic advantage.
All this makes it abundantly clear that robust medical waste disposal and drug diversion prevention are essential, interconnected parts of a safe healthcare waste management program.

Medical Waste Disposal Best Practices
Secure, one-way medical waste containers are a frontline defense against diversion attempts and unauthorized access.
Modern disposal bins, including specialized sharps containers and pharmaceutical waste receptacles, have tamper-proof lids with one-way openings and a secure design that prevents retrieval of discarded drugs or needles once they have been deposited.
These engineered controls provide passive security that works 24/7 without requiring constant supervision. Always use these specialized containers for any unused or expired controlled substances, partial doses that were not administered, contaminated medications, or any pharmaceuticals requiring secure destruction.
Never flush drugs down toilets or sinks under any circumstances. Federal rules explicitly prohibit the disposal of hazardous pharmaceuticals into sewer systems because these substances can pass through wastewater treatment plants unchanged, contaminating drinking water sources, harming aquatic life, and causing ecological damage that persists for years.
Instead, expired or unwanted medications should be deposited into approved pharmaceutical waste receptacles that chemically neutralize the active drug, physically isolate it for incineration, or otherwise render it inaccessible and unusable.
Develop and document clear disposal procedures that leave no room for ambiguity or interpretation. For each type of controlled drug or drug schedule, define a detailed step-by-step process that staff must follow without exception.
These written procedures should specify who is authorized to dispose of medications, what witnessing requirements apply, how to document disposal, where approved containers are located, and what to do if irregularities are discovered.
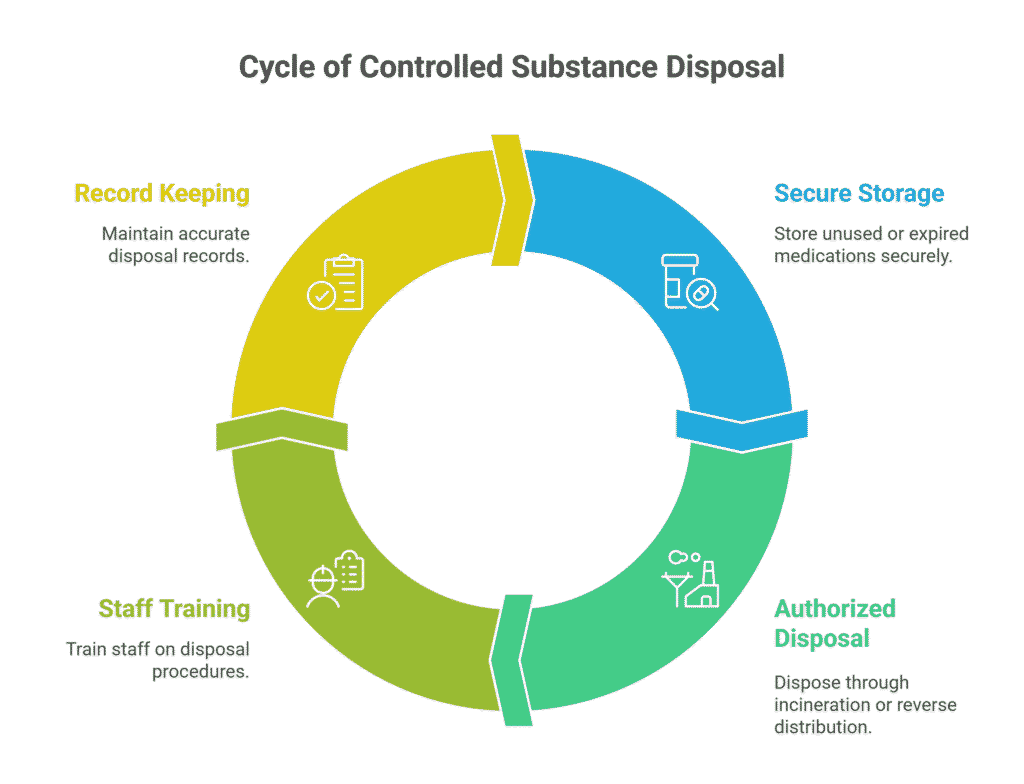
Checklist: Ensuring Safe Controlled Substance Disposal
Maintain accurate, up-to-date inventory logs for all controlled medications in every storage location. Track every dose from delivery to the pharmacy, through dispensing to patient care areas, and finally to administration or disposal. Automated dispensing cabinets can facilitate this tracking, but require diligent use and regular reconciliation. Any gaps in the chain of custody create opportunities for diversion.
Store controlled drugs in locked cabinets or automated dispensing units that limit access to authorized staff only through biometric authentication, PIN codes, or other secure access controls. Multi-factor authentication provides stronger security than single-factor systems. Restrict access to the minimum number of staff necessary for operations, and immediately revoke access when employees change roles or leave the organization.
Use only tamper-evident, one-way disposal containers for unused or expired medications. These containers should be resistant to reopening through force or manipulation and clearly labeled as “DEA Secure Destruction” or similar regulatory language. Position containers in secure areas where disposal activities can be observed, but the containers themselves cannot be easily stolen or tampered with. Please lock medication rooms and treatment areas when they are unattended.
Please make sure to have a witness whenever controlled drugs are discarded.
A second trained staff member should directly observe the disposal, verify the medication and quantity being wasted, and sign off on a log entry that documents the date, time, medication details, reason for disposal, and the identities of both individuals.
This dual-signature requirement adds accountability and transparency, discouraging diversion. The witness should be independent and not have a conflict of interest.
Could you remove any patient identifiers from medication vials, packaging, or syringes before disposing of them to ensure HIPAA privacy protections are maintained?
Scrub labels with permanent markers, remove them physically, or deface them so patient names, medical record numbers, and other protected health information cannot be read. This step prevents privacy breaches when multiple parties handle waste before it is finally destroyed.
Conduct regular audits of disposal records and inventory logs at unpredictable intervals. Check for discrepancies, missing entries, unusual patterns, or variances in drug usage that don’t align with patient census or acuity.
Compare waste rates across similar units or against historical baselines to identify outliers. Investigate any anomalies quickly and thoroughly, treating them as potential diversion until proven otherwise.
Train all staff on these procedures through initial orientation and ongoing education, and update policies annually to reflect lessons learned, new regulations, or technology changes. Training should be competency-based, with an assessment of understanding, rather than just attendance documentation.
Expert Tip:
Establish a cross-department diversion committee with broad representation and apparent authority. Instead of leaving diversion oversight solely to pharmacy staff who may lack visibility into clinical practices, involve representatives from nursing who understand workflow realities, security personnel who can investigate incidents, risk management professionals who understand liability, human resources specialists who handle personnel issues, and administration who can authorize resources.
This multidisciplinary team can share responsibility for monitoring medication use, objectively reviewing incidents, consistently enforcing policies, and creating a culture where diversion is unacceptable. Studies show that facilities with formal diversion committees and specialized software tools have significantly better detection and prevention outcomes compared to facilities relying on informal oversight.
Staff training is a cornerstone of these best practices and cannot be delegated or shortcut.
Every clinician and waste handler should be educated on why diversion is a threat to patient safety and organizational integrity, and how each person can prevent it through vigilance and adherence to procedures.
Training topics should include identifying signs of diversion, such as unusual waste amounts that exceed clinical norms, frequent medication “loss” reports from the same individuals or areas, staff behavior changes, including increased isolation or financial problems, proper administration documentation that creates an audit trail, and exact disposal steps that ensure secure destruction.
Use real-world examples and case studies in training to highlight risks and make the threat tangible rather than abstract.
Could you emphasize OSHA compliance as a parallel safety priority? Under OSHA’s Bloodborne Pathogens Standard, employers are required to train employees who handle potentially infectious or hazardous materials in safety procedures that protect them from occupational exposure to bloodborne pathogens.
This includes correct use of sharps containers and disposal containers to prevent needlestick injuries, appropriate selection and use of personal protective equipment, and procedures for handling spills or exposures.
Unsafe practices in medication disposal can expose staff to injury, infection, or toxic substance exposure, creating liability and workers’ compensation claims.
Ensuring OSHA and HIPAA Compliance
Healthcare waste management is governed by multiple overlapping regulations at the federal and state levels, creating a complex compliance landscape.
OSHA standards require facilities to have written exposure control plans that identify tasks and job classifications with potential exposure, as well as comprehensive staff training on the safe handling of biohazardous or sharps waste.
In practice, this means supplying gloves, eye protection when splash risks exist, and other personal protective equipment (PPE) when disposing of hazardous drugs or contaminated materials. Facilities must provide needle-capping devices, sharps disposal tools positioned at the point of use, and engineering controls that minimize direct handling of dangerous items.
Facilities should also maintain Safety Data Sheets (SDS, formerly MSDS) for pharmaceuticals classified as hazardous under OSHA’s Hazard Communication Standard, and train staff on hazard communication requirements if they work with potent drugs, such as chemotherapy agents or reproductive toxins.
These precautions reduce workplace injuries from chemical exposure, sharp injuries, and other incidents, while ensuring OSHA compliance that protects the organization from citations and penalties.
HIPAA adds another critical layer of compliance that intersects with waste disposal. Any patient information on medication packaging, prescription labels, or administration records must be securely disposed of to prevent unauthorized access and disclosure.
U.S. Department of Health and Human Services guidance makes it clear that covered entities must apply reasonable safeguards to protected health information (PHI) at the disposal stage and may not simply abandon records or labeled items in public trash, where anyone could retrieve them.
This requirement applies to all PHI, regardless of format, including paper, electronic, and physical items such as medication vials with patient labels.
In practice, this means removing or obscuring patient names, medical record numbers, dates of birth, and other identifying codes from any item before it is placed in waste bins accessible to housekeeping or waste contractors.
Many organizations contract with certified secure shredding services or use on-site incineration for printed materials that contain PHI, obtaining certificates of destruction that document compliance. When disposing of actual medications, be sure to deface or completely remove any patient labeling before placing items in pharmaceutical waste containers.
These steps ensure the disposal process complies with HIPAA requirements, protects patient privacy rights, and shields organizations from costly data breach notifications and penalties.
Sustainable and Compliant Disposal Practices
Beyond security and compliance, disposal methods should be environmentally responsible and aligned with broader sustainability goals.
Flushing drugs not only risks water pollution with pharmaceuticals that can harm aquatic ecosystems and potentially enter drinking water supplies, but is explicitly banned by federal regulations.
The EPA and DEA prohibit the disposal of hazardous waste pharmaceuticals, including most controlled substances, into sewer systems through any route. Likewise, tossing medications in regular trash can allow scavenging by individuals or animals, environmental contamination as drugs leach from landfills, and exposure of waste handlers to toxic substances.
Instead, use EPA-approved methods that ensure destruction. The most common approach is incineration at licensed facilities equipped with pollution control technology, which destroys pharmaceuticals at high temperatures.
However, incineration has environmental impacts, including greenhouse gas emissions, air pollutants, and energy consumption. Recognizing these concerns, in 2023, the DEA sought public input on alternatives to incineration for controlled substances, signaling openness to new technologies.
Emerging methods, such as chemical neutralization that renders drugs non-retrievable, high-heat compaction that destroys the molecular structure, and other innovative technologies, could soon offer greener options that meet security requirements while reducing environmental footprints.
National drug take-back programs also support sustainability by providing safe disposal pathways.
The DEA’s National Prescription Drug Take Back Day in October 2023 collected nearly 600,000 pounds of unwanted medications at thousands of community collection sites, preventing these pharmaceuticals from being diverted or entering the environment.
Additionally, almost 17,000 permanent collection receptacles, also known as “drop boxes,” are located in pharmacies, law enforcement facilities, and hospitals nationwide, providing convenient on-demand disposal for patients and their families.
While these events primarily target consumer medicines in homes, the principle applies equally to healthcare facilities: remove unneeded drugs safely before they can cause harm.
Healthcare facilities can encourage patients and their families to utilize these programs upon discharge.
They can host pharmacy take-back drives in their communities as a form of public health outreach. These efforts reinforce sustainable waste practices by ensuring that drugs are rendered unusable and do not enter the environment through water systems, landfills, or diversion channels.
Partnering for Secure Waste Removal
Implementing these comprehensive policies internally is significantly easier with the right partner providing expertise and infrastructure.
Many healthcare providers contract with licensed medical waste disposal firms to handle controlled substance waste, biohazardous materials, and other regulated waste streams.
These specialized companies supply compliant containers designed to meet DEA and EPA requirements, manage scheduled pickups that prevent accumulation, and oversee final destruction at authorized facilities.
Working with a qualified vendor can simplify OSHA compliance, as the provider’s trained and insured staff handle transportation and destruction, ensuring HIPAA rules are followed through secure chain-of-custody documentation.
Choose a provider that offers flexible service without long-term contracts that lock you in regardless of performance, transparent pricing with no hidden fees for fuel surcharges or container rentals, and complete documentation, including manifests, certificates of destruction, and training materials.
For example, a local, bonded medical waste company will tailor pickup schedules to your facility’s waste generation patterns and handle all regulatory paperwork, freeing your staff to focus on clinical care.
By outsourcing secure waste removal to specialists, facilities reduce internal administrative burden and liability exposure. Staff only need to place waste into the provided containers following basic protocols.
The disposal company ensures that the drugs are incinerated or destroyed in accordance with regulatory standards, handles transportation in compliance with DOT hazardous materials regulations, and maintains documentation to demonstrate compliance with these regulations.
This partnership often improves sustainability as well, since reputable waste companies use EPA-compliant incinerators with modern pollution controls and may pursue newer destruction technologies as they become commercially available.
In short, a professional waste removal service becomes an extension of your compliance program, providing peace of mind and allowing you to keep your focus squarely on patient care.
Conclusion
Effective medical waste disposal is crucial for preventing drug diversion and maintaining a safe healthcare environment for patients, staff, and the broader community.
By combining strong policies, including rigorous inventory control and witnessed disposal, with comprehensive staff training and strict regulatory compliance, facilities protect patients from compromised care and personnel from the disease of addiction.
Sustainable waste practices, such as using one-way containers, avoiding environmentally harmful flushing, and supporting community take-back programs, further safeguard public health and environmental quality.
Secure Waste is ready to help implement these solutions with expertise and reliability. Our team offers compliant, cost-effective disposal services with flexible scheduling tailored to your needs, and there are no hidden fees or long-term obligations. To learn more about secure medical waste removal and to request a free waste management assessment customized to your facility, visit securewaste.net today.

Expert Medical Waste Management: With over 25 years of industry experience, Secure Waste is a trusted local leader in hazardous and biohazardous waste disposal across Maryland, Virginia, and Washington, D.C. Specializing in medical waste management, sharps needle disposal, and biohazard waste removal, the company ensures full compliance with federal, state, and local regulations while prioritizing environmental sustainability.
The company also offers additional services, including secure document shredding and sharps container sales, providing comprehensive solutions for healthcare facilities and businesses. Our cost-effective services help clients maintain regulatory compliance without unexpected costs.
With a commitment to customer satisfaction, Secure Waste offers tailored waste management plans that align with industry best practices. Their team of experts provides reliable, timely, and compliant services, making them the preferred choice for medical waste disposal. For a free waste quote or more information, visit www.securewaste.net






