HIPAA Explained For Healthcare Organizations
Welcome to Secure Waste, a recognized leader in biohazard disposal services, proudly serving Maryland, Virginia, and Washington, D.C. With over a decade of experience in healthcare waste management, we are committed to delivering innovative and customized solutions that meet the diverse and evolving needs of our clients.
Our comprehensive suite of services encompasses the safe collection, transportation, treatment, and disposal of a wide range of medical waste, including sharps (such as needles and blades), pharmaceuticals (expired or unused medications), and microbiological waste (cultures and specimens). We utilize state-of-the-art technology and adhere to industry best practices to ensure that all waste is handled safely and efficiently, mitigating any potential environmental or health risks.
In addition to our disposal services, we place a strong emphasis on HIPAA (Health Insurance Portability and Accountability Act) compliance. Our experienced team of professionals is adept at guiding healthcare providers through the complex regulatory landscape that governs patient privacy and data security. We offer personalized training sessions, easy-to-understand resources, and ongoing support to ensure your organization remains compliant, safeguards sensitive information, and minimizes legal risks.
By partnering with Secure Waste, you can redefine and streamline your waste management processes, ensuring operational efficiency and regulatory compliance across Maryland, Virginia, and Washington, D.C. Trust in our expertise to help you maintain high standards of safety and efficiency, protecting both your organization’s reputation and the confidentiality of your patients’ information. Let us take the burden of waste management off your shoulders, so you can focus on delivering exceptional care to your patients.
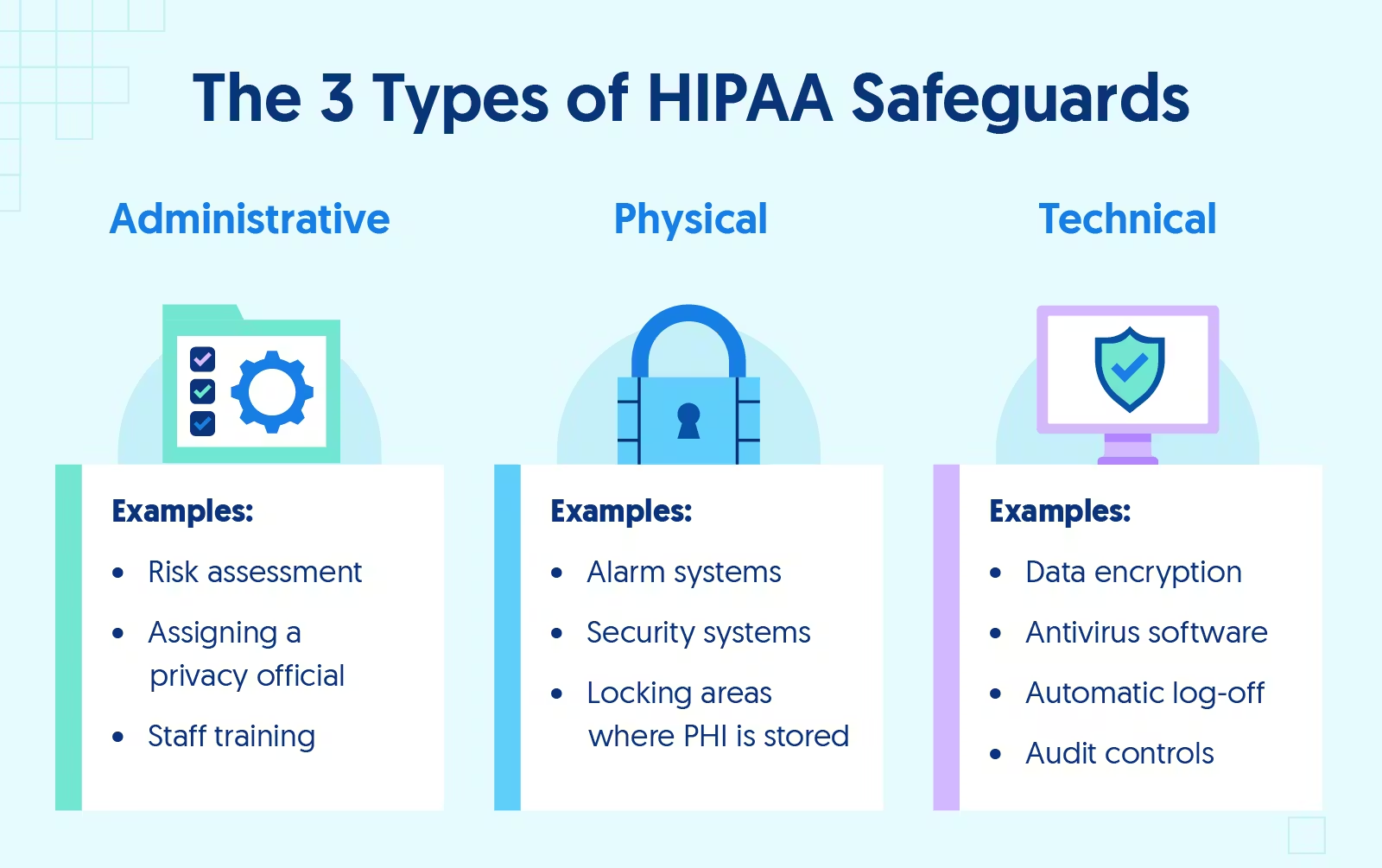
Navigating the complexities of the Health Insurance Portability and Accountability Act HIPAA can be challenging for healthcare organizations This guide addresses common questions and concerns related to HIPAA compliance in Maryland Washington DC and Virginia providing essential information to help organizations understand their responsibilities and ensure the protection of patient information
HIPAA FAQs Explained for Healthcare Organizations
Compliance with the Health Insurance Portability and Accountability Act remains one of the most complex and misunderstood responsibilities facing healthcare organizations. HIPAA governs the creation, access, sharing, storage, and destruction of protected health information. Its scope applies to organizations of all sizes, from large hospital systems to dental offices and specialty practices. Confusion often arises not because organizations disregard compliance, but because the law involves overlapping rules, evolving technologies, and nuanced exceptions.
Understanding how HIPAA applies in real-world scenarios is essential to building a reliable compliance framework. The most common questions tend to center on information disclosure, patient rights, data security risks, and the use of electronic communications. Addressing these areas clearly helps organizations reduce risk while maintaining efficient patient care operations.
Handling Patient PHI Requests Versus Third-Party Requests
One of the most frequent sources of HIPAA confusion involves determining when protected health information can be released, to whom, and under what conditions. Patient requests for their own information are treated differently from requests made by outside entities such as attorneys, insurers, employers, or other healthcare providers.
Patients have a legal right to access their own medical information. Healthcare organizations are expected to facilitate this access without unnecessary barriers. Processes should be straightforward, timely, and clearly documented. Patients should be able to request copies of their information and direct where those records are sent when needed for personal, legal, or medical reasons.
Requests from third parties require additional scrutiny. Unlike patient requests, these disclosures often require written authorization unless a specific HIPAA exception applies. Organizations must have documented procedures for evaluating these requests and verifying that disclosure is permitted. Staff should know where authorization forms are stored, how to confirm their validity, and when to deny or limit requests that do not meet regulatory standards.
Responding to Pharmacy Requests for Patient Information
Pharmacy disclosures represent a unique compliance challenge. Pharmacies frequently request patient information to verify prescriptions, confirm medication histories, or comply with controlled substance regulations. While HIPAA allows disclosures for treatment purposes, organizations must still ensure that information is shared appropriately and securely.
The key to compliance in these situations is balance. Excessive restrictions can delay patient care and pose safety risks, while insufficient controls may lead to over-disclosure. Clear internal policies should define which information can be shared, how identity verification is conducted, and which staff members are authorized to respond to pharmacy inquiries.
When uncertainty arises, direct communication between healthcare providers and pharmacists is often the most effective solution. Clear, professional dialogue supports patient safety while maintaining regulatory compliance.
Commonly Overlooked HIPAA Vulnerabilities
Data security vulnerabilities remain a leading cause of HIPAA enforcement actions. Many organizations focus on internal safeguards but overlook risks introduced by third-party vendors and business associates. Any external partner that handles protected health information must meet HIPAA security standards.
Healthcare organizations are responsible for ensuring that business associates maintain adequate protections. This includes having executed agreements, verifying security practices, and conducting periodic reviews. A vendor’s failure can result in reputational harm, patient distrust, and regulatory consequences for the covered entity.
Physical data security is another commonly underestimated risk. Paper records, discarded files, unattended printers, and unsecured storage areas continue to contribute to privacy incidents. HIPAA compliance requires attention to both electronic and physical information pathways.
HIPAA and Electronic Communications
Electronic communication with patients has become increasingly common, but it introduces compliance considerations that organizations must manage carefully. HIPAA applies to any communication that includes protected health information, regardless of format.
Email and secure patient portals are generally permitted, provided appropriate safeguards are in place. Encryption is strongly recommended, though not always mandatory. At a minimum, organizations should document patient consent for electronic communication and confirm the accuracy of contact information.
Text messaging and social media platforms pose higher risks due to limited security controls and should be avoided for sharing protected information. Staff training is critical to ensure employees understand which communication channels are approved and how to use them properly.
Managing Emerging Technologies and Patient Data
Healthcare technology continues to evolve rapidly, introducing new compliance challenges. Mobile health applications, remote monitoring tools, and patient portals are increasingly integrated with electronic health records. While these technologies improve efficiency and patient engagement, they also create new data flows that must be evaluated under HIPAA.
Organizations should assess how new tools collect, transmit, and store patient information. Risk assessments should be conducted before implementation, and policies should be updated as technologies change. Ongoing monitoring ensures that compliance keeps pace with innovation.
Preparing for HIPAA Investigations
HIPAA investigations may occur in response to patient complaints, reported breaches, or regulatory audits. The scope of an investigation varies, ranging from document requests to onsite assessments. Organizations must be prepared to demonstrate compliance through policies, training records, and risk assessments.
Failure to provide adequate documentation can result in corrective action plans, fines, or increased regulatory oversight. Maintaining up-to-date policies and conducting regular internal reviews significantly reduces exposure during investigations.
Training as the Foundation of Compliance
Employee training is a cornerstone of HIPAA compliance. Staff must understand their role in protecting patient information, recognizing risks, and responding to incidents. Training should be provided to new hires and reinforced regularly through refresher sessions.
Practical training covers the handling of verbal, paper, and electronic information. It also addresses real-world scenarios employees are likely to encounter. Documenting training completion is essential for demonstrating compliance.
Building a Sustainable HIPAA Compliance Program
HIPAA compliance is not a one-time task but an ongoing operational commitment. Organizations that succeed in maintaining compliance integrate privacy and security into daily workflows. Clear policies, defined responsibilities, regular audits, and leadership support all contribute to long-term success.
Secure Waste supports healthcare organizations by providing compliance-focused solutions designed to reduce risk and protect sensitive information. Learn more at https://www.securewaste.net/.
Final Takeaway
HIPAA compliance requires clarity, consistency, and proactive management. By understanding how to handle PHI requests, manage pharmacy disclosures, secure electronic communications, and address overlooked vulnerabilities, healthcare organizations can build a resilient compliance framework. A structured approach protects patients, strengthens trust, and supports safe, efficient care delivery across all practice settings.

Expert Medical Waste Management: With over 25 years of industry experience, Secure Waste is a trusted local leader in hazardous and biohazardous waste disposal across Maryland, Virginia, and Washington, D.C. Specializing in medical waste management, sharps needle disposal, and biohazard waste removal, the company ensures full compliance with federal, state, and local regulations while prioritizing environmental sustainability.
The company also offers additional services, including secure document shredding and sharps container sales, providing comprehensive solutions for healthcare facilities and businesses. Our cost-effective services help clients maintain regulatory compliance without unexpected costs.
With a commitment to customer satisfaction, Secure Waste offers tailored waste management plans that align with industry best practices. Their team of experts provides reliable, timely, and compliant services, making them the preferred choice for medical waste disposal. For a free waste quote or more information, visit www.securewaste.net






